Lipidomics on nanoneedles
We assessed the suitability of nanoneedles for DESI-MSI lipidomics, utilizing nanoneedle arrays with 2 μm pitch, 4.00 μm peak, 50 nm tip diameter, 600 nm base diameter, 77.2 m2 g−1 floor space, 50% porosity and seven.9 nm common pore diameter, on 8 × 8 mm silicon substrates (Supplementary Fig. 1). The evaluation of porcine mind lipid extract revealed that nanoneedles and flat surfaces supply comparable sensitivity for essentially the most consultant lipids, according to established DESI limits of detection (LODs)19 (Prolonged Information Fig. 1a,b and Supplementary Desk 1). Nanoneedle substrates usually displayed barely increased LODs than flat silicon, suggesting that nanotextured surfaces can dampen the desorption–ionization course of. Nevertheless, the relative peak depth correlated properly between nanoneedles and flat substrates, indicating that the evaluation on nanoneedles precisely recapitulated the lipid composition of the combination (Prolonged Information Fig. 1c and Supplementary Fig. 2). Optimization of DESI-MSI analytical parameters20 for nanoneedles considerably elevated the variety of detectable options by an element of two.47, from 408 ± 83 to 1,007 ± 141 (Prolonged Information Fig. 1d–f).
Lipidomics imaging of molecular replicas
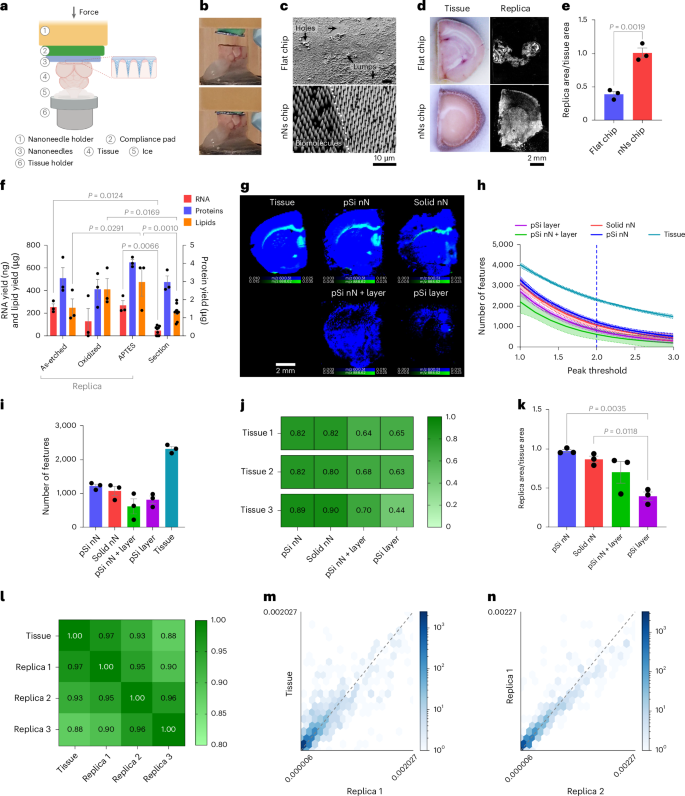
We then established the flexibility of nanoneedles to generate a molecular duplicate by imprinting frozen mouse mind (Fig. 2a,b). Computational modelling indicated that, throughout imprinting, a couple of micrometres of tissue transiently thaw, permitting assortment of biomolecules (Supplementary Fig. 3). The ensuing duplicate shaped a uniform molecular adsorbate on the nanoneedles, whereas utilizing a flat substrate yielded poor adsorbate uniformity, presenting areas with out protection and areas of adsorbate accumulation (Fig. 2c). The nanoneedle duplicate absolutely reproduced the unique tissue and macroscopically retained its morphology, whereas the flat duplicate reproduced solely lower than half of the tissue, missing clear morphology (Fig. 2nd,e).
Fig. 2: Lipidomics imaging of molecular replicas.
a, Schematics of the nanoneedle imprinting system. Created with BioRender.com. b, Images illustrating the system approaching (high) and imprinting (backside) nanoneedles on tissue. c, Scanning electron microscopy photos of the molecular adsorbate on flat and nanoneedle substrates after imprinting. d, Images of the imprinted tissue and fluorescence microscopy photos of the corresponding molecular duplicate. e, Quantification of replica-to-tissue space ratio. Information are imply ± s.e.m. (N = 3 impartial organic replicates). Statistics: two-tailed unpaired t-test. f, Quantification of RNA, lipids and proteins from nanoneedle replicas with totally different floor chemistries (as-etched, APTES, oxidized) and a 10-µm mind tissue part. ata are imply ± s.e.m. (N = 3 impartial organic replicates). Statistics: two-way ANOVA with a post-hoc Tukey’s multiple-comparisons check. g, DESI-MSI maps of gray matter (m/z 834.53, blue) and white matter (m/z 888.62, inexperienced) lipids from a tissue slice and molecular replicas generated utilizing porous silicon nanoneedles (pSi nN), stable silicon nanoneedles (stable nN), porous silicon nanoneedles over a porous silicon layer (pSi nN + layer) and a porous silicon layer (pSi layer). h, Quantification of the variety of options as a operate of the arrogance threshold set for peak identification in samples from g. Information are imply ± s.e.m. (N = 3 impartial organic replicates). i, The function depend at a peak threshold of two.0 (95% confidence) for samples from g. Information are imply ± s.e.m. (N = 3 impartial organic replicates). j, The correlation matrix of spectra from three tissue sections and their corresponding replicas from g. okay, The relative floor space of molecular replicas to unique tissue for samples from g. Information are imply ± s.e.m. (N = 3 impartial organic replicates). Statistics: peculiar one-way ANOVA with post-hoc Tukey’s multiple-comparisons check. l, A correlation matrix evaluating three pSi nN replicas with the unique tissue part. m, A correlation scatter plot of lipids peak intensities between the tissue part and the primary duplicate. n, A correlation scatter plot of lipid peak intensities between the primary and second duplicate.
Supply knowledge
We then evaluated the composition of the molecular adsorbate on replicas generated from nanoneedles as-etched, O2-plasma oxidized and (3-aminopropyl)-triethoxysilane (APTES) functionalized, assessing the function of floor polarity and cost on biomolecular assortment efficiency21,22. Oxidation and APTES respectively generated unfavorable and optimistic fees, offering extra polar surfaces than the as-etched porous silicon. For all of the functionalizations thought of, the quantity of collected RNA, proteins and lipids in contrast favourably with people who could possibly be extracted from the reference tissue (Fig. 2f). RNA assortment ranged between 210 ng and 350 ng, whereas protein assortment ranged between 2.5 μg and 4.3 μg. Lipid assortment was extra environment friendly on APTES and oxidized nanoneedles in contrast with as-etched ones, ranging between 200 μg and 600 μg. These knowledge indicated that plasma oxidized nanoneedles have been appropriate for spatial lipidomics, as a result of their lipid assortment effectivity of 410 ± 95 μg was properly throughout the vary through which numerous options have been detectable (Fig. 2f) and in contrast favourably with a 10-μm tissue part (210 ± 23 μg), identified to be imageable by DESI-MSI. Using oxidized nanoneedles additionally mitigated potential issues relating to interference from the desorption of polymerized APTES.
We additionally in contrast the standard of the duplicate generated utilizing porous silicon nanoneedles (pSi nN) with these obtained utilizing stable silicon nanoneedles (stable nN), porous silicon nanoneedles over a steady porous silicon layer (pSi nN + layer) and a steady porous silicon layer (pSi layer)23,24,25 (Supplementary Fig. 4). pSi nN displayed the most important variety of options and the least variability, adopted by the stable nN, whereas the pSi nN + layer and pSi layer yielded much less options with increased variability (Fig. 2g–i). The pSi nN additionally outperformed the opposite substrates of their spectral correlation to the unique tissue, carefully matched by stable nN (Fig. 2j), and most carefully reproduced the floor space of the unique tissue (Fig. 2g,okay).
The imprinting was reproducible, with three replicas from the identical floor preserving morphological options and displaying sturdy correlation with the reference part and with one another throughout the entire vary from low- to high-abundance lipids (Fig. 2l–n and Supplementary Fig. 5).
These knowledge indicated {that a} molecular duplicate generated utilizing porous silicon nanoneedles may precisely and robustly reproduce the lipidomic profile of a tissue. Nevertheless, the presence of a steady porous layer lowered the effectivity of the desorption–ionization course of, favouring the usage of substrates with nanoneedles solely. As well as, porous nanoneedles marginally outperformed stable ones, producing replicas that extra carefully represented the unique tissue by way of space, variety of options and correlation.
Spatial lipidomics of murine glioma replicas
We in contrast the standard of lipidomic imaging on molecular replicas with its proximal tissue section5. Hierarchical cluster evaluation (HCA) revealed that the highest two clusters of each part and duplicate appeared to align with the areas of gray and white matter throughout the mouse mind structure (Fig. 3a). The white matter cluster aligned with mind areas containing extremely myelinated fibres, matching the expression sample of myelin genes akin to myelin primary protein in areas such because the corpus callosum. In the meantime the gray matter cluster corresponded to areas with increased cell physique densities and fewer myelinated fibres, such because the cortex4.
Fig. 3: Spatial lipidomics of murine glioma replicas.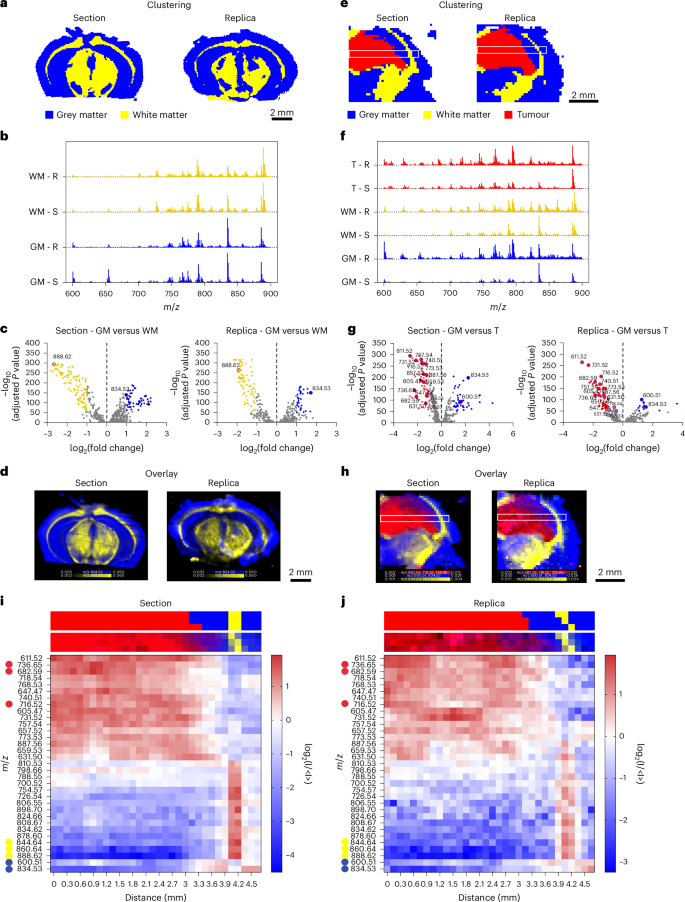
a, HCA maps of the highest two clusters from the DESI-MSI knowledge of a murine mind part and its molecular duplicate, comparable to gray matter (blue) and white matter (yellow). b, Common spectra displaying normalized counts versus m/z, for the highest two clusters related to gray matter (GM) and white matter (WM) in part (S) and duplicate (R). c, Volcano plots of the differential lipid distribution between gray and white matter, highlighting the function of m/z 834.53 (PS 40:6) and 888.62 (SHexCer 42:2;O2) as gray and white matter biomarkers in each part and duplicate. d, An overlay map of TIC-normalized depth for m/z 834.53 (blue) and 888.62 (yellow). e, HCA maps of the highest three clusters from the DESI-MSI dataset of a tumour-bearing mouse mind part and duplicate, comparable to gray matter (GM, blue), white matter (WM, yellow), and tumour (T, crimson). White containers point out areas used for lipid abundance evaluation. f, Common spectra displaying normalized counts versus m/z, for the three clusters related to gray matter (GM), white matter (WM) and tumour (T) in part (S) and duplicate (R). g, Volcano plots evaluating lipid distribution between GM and T, highlighting essentially the most consultant lipid peaks in each part and duplicate. h, An overlay map of cumulative TIC-normalized depth for peaks marking gray matter (m/z 834.53 and m/z 600.51, blue), white matter (m/z 844.64, m/z 860.64 and m/z 888.62, yellow) and tumour (m/z 682.59, m/z 716.52 and m/z 736.65, crimson) in part and duplicate. The white containers point out areas used for lipid abundance evaluation throughout GM, WM and T. i,j, Heatmaps displaying the relative abundance of 33 lipid peaks (from evaluation in g) alongside the foremost axis of the white field in e and h for the part (i) and duplicate (j). Prime and backside strips above every heatmap present the cluster task and relative lipid depth for corresponding pixels from e and h.
Supply knowledge
The lipid composition of those two clusters confirmed this morphological classification (Fig. 3b,c). The upper abundance of m/z 888.62 (sulfated hexosylceramide (SHexCer) 42:2;O2) and decrease abundance of m/z 834.53 (phosphatidylserine (PS) 40:6) in white matter have been according to established DESI-MSI mind biomarkers26. Mapping the relative depth of those two peaks throughout the pattern precisely delineated white and gray matter areas in each the duplicate and the reference part (Fig. 3d). The congruent distribution of those lipid species throughout duplicate and part confirmed the feasibility of producing a lipidomic profile map from a nanoneedle duplicate of the mouse mind. Minor discrepancies in mind morphology have been attributed to the inherent anatomical variations between adjoining tissue sections.
We then used the molecular duplicate to characterize a cancerous lesion inside a mouse glioma5. The highest three HCA clusters categorized white matter, gray matter and tumour areas with comparable effectiveness in part and duplicate (Fig. 3e). When analysing a dataset together with all spectra throughout tissue and duplicate, HCA equally discriminated tumour, white matter and gray matter, highlighting the correspondence of the lipid profile on duplicate and part. Principal element evaluation of intercluster variance additional confirmed that each the duplicate and the part displayed comparable attribute options, enabling correct mapping of tumour margins and transitions between tissue sorts (Supplementary Fig. 6).
Comparative evaluation of the typical mass spectra for every cluster allowed the lipid compositions of white matter, gray matter and tumour to be distinguished and essentially the most related differentially plentiful species to be recognized (Fig. 3f,g and Supplementary Fig. 7). Once more, clusters related to white matter in each the part and duplicate exhibited a distinguished peak at m/z 888.62 (SHexCer 42:2;O2), whereas the gray matter clusters confirmed a distinguished peak at m/z 834.53 (PS 40:6). The tumour cluster confirmed a rise within the abundance of m/z 736.65 (ceramide (Cer) 46:1;O4), m/z 682.59 (Cer 42:0;O4) and m/z 716.52 (phosphatidylethanolamine (PE) 34:1). The depth map for these attribute species successfully discriminated tumour, white matter and gray matter within the duplicate in addition to within the part (Fig. 3h), demonstrating the duplicate’s capability to precisely reproduce the lipidomic map of the unique tissue, even within the presence of a cancerous lesion. The evaluation of two further, distinct mind samples confirmed the reproducibility of our method (Supplementary Fig. 8).
We assessed the spatial distribution of the 33 most consultant species within the part and duplicate by evaluating their abundance alongside the foremost axis (size) of the white field (Fig. 3h and Supplementary Fig. 9). The heatmaps from each part and duplicate revealed a transparent and congruent sample in lipid abundance throughout tumour, gray matter and white matter (Fig. 3i,j and Supplementary Fig. 9). The tumour–gray matter transition occurred at 3.1 mm from the left edge. The tumour was wealthy in lipids at m/z 736.65 (Cer 46:1;O4), m/z 682.59 (Cer 42:0;O4) and m/z 740.51 (phosphatidylcholine (PC) 30:0), whereas gray matter confirmed excessive ranges of m/z 600.51 (Cer 36:1;O2) and m/z 834.53 (PS 40:6). At 4 mm, a 0.3 mm white matter layer was enriched in m/z 844.64 (PS O-41:1), m/z 860.64 (PS 41:0) and m/z 888.62 (SHexCer 42:2;O2) adopted by gray matter (4.3–4.7 mm) with a lipid profile according to the earlier gray matter area.
These knowledge point out {that a} molecular duplicate can map the biomolecular structure of a tumour-bearing tissue. The standard of the duplicate info allows unsupervised classification of particular person pixels with comparable constancy to the unique pattern enabling mapping lipid abundance with comparable high quality to the tissue.
Spatial lipidomics of HG duplicate
We then captured the molecular heterogeneity of a HG biopsy. Histopathological evaluation recognized three distinct areas of wholesome mind infiltrated by tumour (I), bulk tumour (T) and necrosis (N) (Fig. 4a). HCA may distinguish these three areas and establish their lipid composition within the duplicate and the part (Fig. 4b–e). Mapping the distribution of the height at m/z 600.51 (Cer 36:1;O2) characterised the infiltrated tumour, whereas m/z 888.62 (SHexCer 42:2;O2) distinguished the majority tumour and m/z 680.54 (Cer 43:0;O3) preferentially related to areas of necrosis (Fig. 4f).
Fig. 4: Spatial lipidomics of HG duplicate.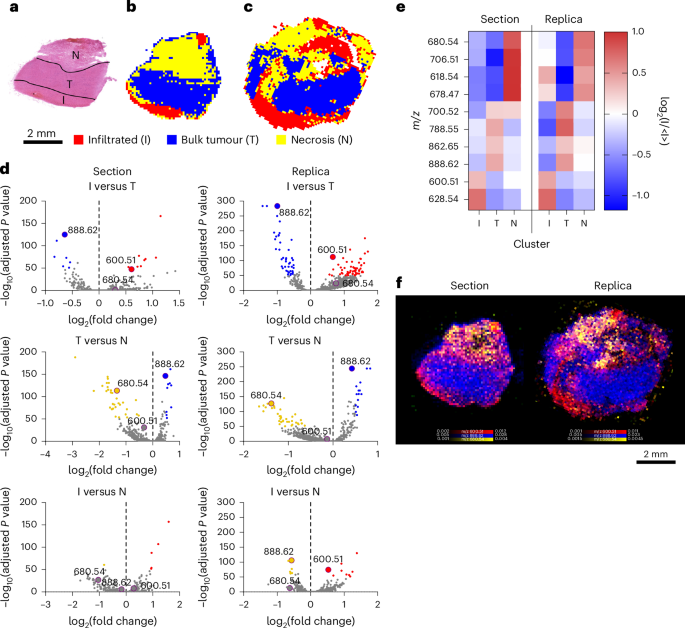
a, A bright-field picture of the haematoxylin and eosin (H&E)-stained part of HG biopsy, displaying infiltrated tumour (I), bulk tumour (T) and necrosis (N). b,c, HCA maps of the highest three clusters from DESI-MSI photos of the part (b) and duplicate (c). d, Volcano plots displaying the differential lipid abundance evaluation throughout I, T and N. persistently figuring out m/z 600.51 (Cer 36:1;O2) for I, m/z 888.62 (SHexCer 42:2;O2) for T and m/z 680.54 (Cer 43:0;O3) for N in part and duplicate. e, A heatmap of the ten most consultant lipids defining I, T and N in each part and duplicate. f, An overlay map of TIC-normalized depth for m/z 600.51 (Cer 36:1;O2, crimson), 888.62 (SHexCer 42:2;O2, blue) and 680.54 (Cer 43:0;O3, yellow).
Supply knowledge
Machine studying inference of HG grade
To guage whether or not replicas may match the predictive energy of unique tissues, we collected DESI-MSI maps of molecular replicas (HG-r) and reference sections (HG-s) from 23 HG samples with identified prognoses (Supplementary Desk 2). Extra sections (HG 11, 12) and replicas (HG 6, 18) have been collected for intrapatient and interreplica validations, for a complete of 25 sections and 25 replicas.
We noticed a excessive correlation between replicas and their reference tissue part, indicating a preserved molecular profile (Fig. 5a). The correlation with the reference part ranked first with a likelihood of 0.85, and ranked within the high 5 with a likelihood of 0.96, properly above the random expectations of 0.04 and 0.2, respectively (Fig. 5b). Replicas from the identical part preserved a standard molecular signature, displaying increased correlation with their reference part than with another (HG 6, 18) (Fig. 5c).
Fig. 5: Machine studying inference of HG grade.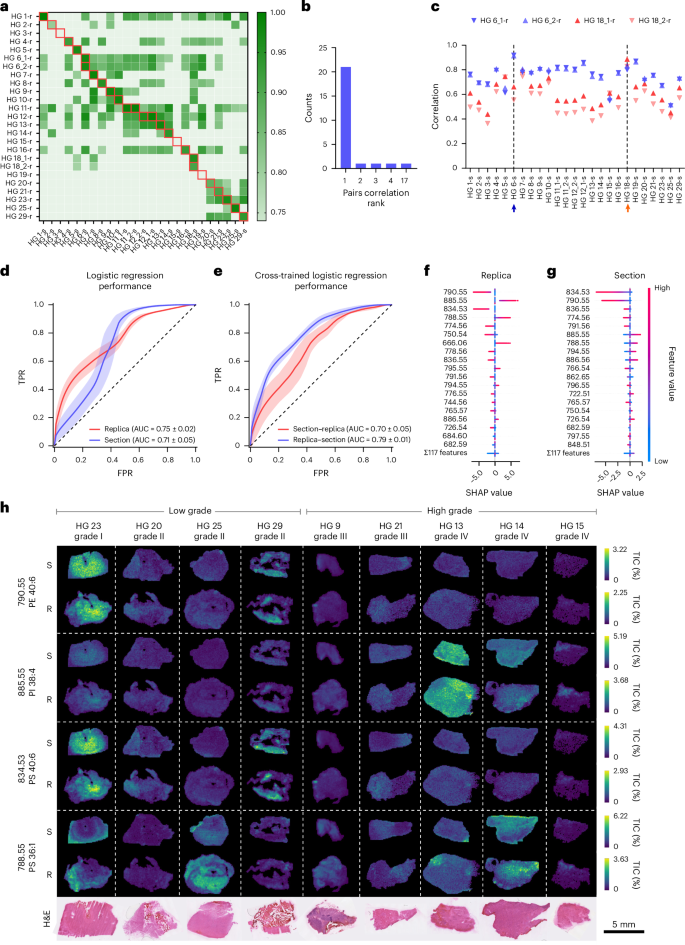
a, A heatmap of correlation values between 25 tissue sections and 25 replicas from 23 biopsy samples. Samples are labelled HG plus a sequential quantity; a number of sections or replicas from the identical biopsy embody a trailing underscore and quantity. Sections are marked with ‘s’ and replicas with ‘r’. The crimson containers point out matched part–duplicate pairs. b, A depend plot of correlation rank between matching tissue sections and molecular replicas. c, Pearson correlation evaluation of the molecular replicas HG6_1-r, HG6_2-r, HG18_1-r and HG18_2-r, with all 25 sections. The arrows mark the reference HG6-s (blue) and HG18-s (orange) part. d, Receiver operator traits for the logistic regression classifier utilized to the HG dataset. Information are imply ± s.d. from 100 random-seed classifications. e, Receiver operator traits for logistic regression cross-inference: mannequin educated on sections, examined on replicas and vice versa. Information are imply ± s.d. from 100 random-seed classifications. TPR, true optimistic charge; FPR, false optimistic charge. f,g, SHAP worth distributions of top-ranked options within the duplicate (f) and the part mannequin (g). Optimistic SHAP values point out contribution to high-grade predictions. h, TIC-normalized ion maps of chosen replicas (R) and tissue sections (S) displaying the highest 4 peaks from f, alongside H&E-stained morphology.
Supply knowledge
We subsequent assessed illness grade prediction utilizing sections and replicas evaluating 4 classification fashions: logistic regression, resolution bushes, XGBoost27 and LightGBM28. All fashions carried out higher than a random mannequin for each sections and replicas (Fig. 5d and Supplementary Fig. 10), with illness grade assignments displaying a excessive diploma of concordance. Logistic regression demonstrated superior efficiency (Fig. 5d and Supplementary Fig. 10) and thus was thought of for additional evaluation. The classification efficiency was comparable for sections and replicas with a imply (±s.d.) space below the curve (AUC) for the receiver working attribute (ROC) of 0.71 ± 0.05 and 0.75 ± 0.02, respectively (Fig. 5d and Supplementary Desk 3). Bootstrapping statistical validation confirmed that the noticed classification efficiency was not because of probability (Supplementary Fig. 11). The very best-performing fashions agreed on 25 out of the 27 part–duplicate pairs, additional indicating that the fashions captured comparable discriminative info from the spectral knowledge (Supplementary Desk 2). Cross-model prediction evaluation making use of the fashions educated on sections to foretell the replicas achieved an AUC of 0.79 ± 0.01, whereas fashions educated on replicas to foretell the sections achieved an AUC of 0.70 ± 0.05, additional supporting the similarity of their molecular profiles (Fig. 5e and Supplementary Desk 3). Shapley additive explanations (SHAP)29 confirmed that part and duplicate shared three of the highest 5 most predictive peaks, whereas 9 out of the highest ten displayed the identical correlation with lesion grade (Fig. 5f,g). Among the many high 4 duplicate peaks, two (m/z 790.55 (PE 40:6) and m/z 834.53 (PS 40:6)) correlated with low-grade tumours and two (m/z 885.55 (phosphatidylinositol (PI) 38:4) and m/z 788.55 (PS 36:1)) with excessive grade. Their depth maps throughout consultant tumours aligned with anticipated tumour grades (Fig. 5h). This evaluation additionally captured the spatial heterogeneity of tumours, figuring out the decrease part of HG 25—histologically infiltrated wholesome mind—as low grade, enriched within the m/z values of 790.55 and 834.53, and the sides of HG 23 as excessive grade, enriched in m/z 788.55 and depleted in low-grade markers. These outcomes established that the molecular duplicate generated utilizing nanoneedles can change a tissue part for illness state classification.
Molecular replicas of dwelling tissues
The non-destructive molecular assortment enabled by nanoneedles provides a singular alternative to pattern dwelling tissue longitudinally, permitting one to trace its spatiotemporal lipidomic evolution. We examined this capability by monitoring the response of reside glioma-bearing tissue slices to temozolomide (TMZ) therapy. We collected longitudinal molecular replicas on day 0 (D0T1, handled; D0T0, management) and day 5 (D5T1, handled; D5T0, management) whereas exposing the samples to TMZ or dimethylsulfoxide from day 3 (Fig. 6a).
Fig. 6: Spatiotemporal lipidomics of molecular replicas.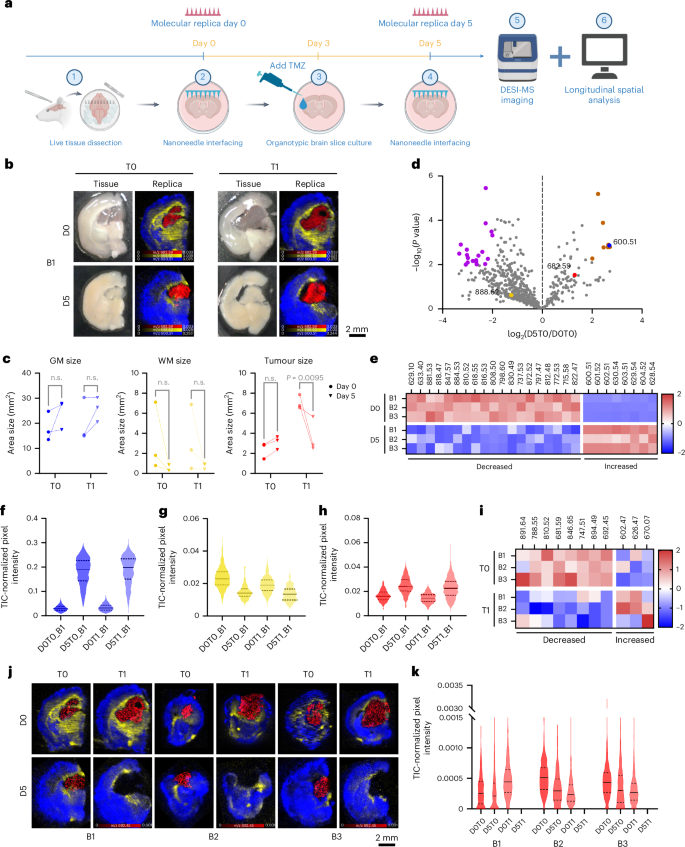
a, A schematic of the longitudinal evaluation workflow. Created with BioRender.com. b, Images (left) of mind 1 (B1) displaying handled (T1) and untreated (T0) tissue slices at day 0 (D0) and day 5 (D5), with corresponding DESI-MSI maps (proper) of gray matter (m/z 600.51, blue), white matter (m/z 888.62, yellow) and tumour (m/z 682.59, crimson) markers from molecular replicas of the photographed acute tissue slice. c, Quantification of gray matter, white matter and tumour areas from DESI-MSI molecular replicas knowledge throughout time and therapy in three mind slices. Statistics: peculiar two-way ANOVA with post-hoc Šidák multiple-comparisons check. n.s., not vital. d, A volcano plot of lipid adjustments over time in untreated brains, highlighting markers for white matter (yellow), gray matter (blue) and tumour (crimson) and considerably elevated (brown) or decreased (purple) species. N = 3 mind slices. e, A heatmap of the differentially plentiful species (orange and purple) proven in d. f–h, Violin plots of gray matter (f), white matter (g) and tumour (h) marker abundance in mind 1 throughout time and therapy. Information: stable line: median; dashed strains: higher and decrease quartiles. i, A heatmap of z scores for treatment-dependent vital lipids. j, DESI-MSI maps of handled and untreated molecular replicas at day 0 and day 5 for the lipid at m/z 692.45 (PS 29:0, crimson) within the tumour alongside gray matter (m/z 600.51, Cer 36:1;O2, blue) and white matter (m/z 888.62, SHexCer 42:2;O2, yellow) markers. okay, Violin plots of m/z 692.45 (PS 29:0) abundance. Information: stable line: median; dashed strains: higher and decrease quartiles.
Supply knowledge
We first assessed the feasibility of producing molecular replicas from dwelling tissue with minimal perturbation. Nanoneedle imprinting didn’t influence mind slice viability instantly (Prolonged Information Fig. 2a,b) or over the 5 days of tradition (Prolonged Information Fig. 2c,d), whereas the TMZ therapy selectively lowered the viability of cells within the tumour area by day 5 (Prolonged Information Fig. 2e–g). The day 0 and day 5 replicas captured your entire tissue slice space (Prolonged Information Fig. 2h).
Spatiotemporal lipidomics of molecular replicas
We used these longitudinal molecular replicas to check the spatiotemporal variations within the lipidomic profile in response to TMZ therapy. The DESI imaging of the molecular replicas at each timepoints confirmed well-defined mind areas (Fig. 6b and Supplementary Fig. 12a,c). Tumour, gray matter and white matter have been distinguished by the identical lipids recognized for the frozen sections (Figs. 3h and 6b). The morphology of those areas aligned properly with the anticipated construction of the tumour-bearing mind and carefully matched the mind construction noticed within the slices, which advanced over the 5 days in tradition. Over time, and no matter therapy, the dimensions of the gray matter barely elevated and the white matter decreased, according to the anticipated axonal degeneration in cultured mind slices30,31 (Fig. 6c). In contrast, the tumour dimension barely elevated in untreated samples whereas lowering considerably in response to TMZ therapy, supporting the remark that the therapy selectively focused tumour cells (Fig. 6c and Prolonged Information Fig. 2g).
We then assessed the temporal evolution of lipid composition by evaluating lipid abundance between untreated samples at day 0 (D0T0) and day 5 (D5T0) throughout the three brains. Among the many 629 generally recognized lipids on day 0, 21% elevated in abundance over time, whereas 79% decreased with 31 of them changing into undetectable (Fig. 6d). Amongst these, 27 lipids confirmed vital adjustments, with 8 rising and 19 lowering in abundance (Fig. 6e). The white matter and tumour markers didn’t range considerably, whereas the gray matter marker abundance elevated over time (Fig. 6f–h and Supplementary Fig. 12b,d). The information point out an general lower within the abundance and variety of species as a consequence of culturing the mind slices. This evaluation captures mind tissue remodelling throughout culturing and divulges adjustments in lipid composition and spatial distribution inside particular person slices, insights made attainable via longitudinal sampling enabled by nanoneedles.
We then analysed the impact of therapy particularly throughout the tumour. Total, the relative abundance of 42% of lipids elevated with therapy, whereas 58% decreased. Statistical evaluation recognized 11 vital lipids (Fig. 6i), with 8 lowering and three rising in abundance with TMZ therapy. Essentially the most vital, m/z 692.45 (PS 29:0), selectively disappeared in handled tumours throughout all samples (Fig. 6j,okay). Different lipid species confirmed distinct, treatment-dependent abundance patterns, highlighting the facility of longitudinal molecular duplicate evaluation to detect lipid-specific and specimen-specific therapy responses (Supplementary Fig. 13). Phosphatidylserines (PS29:0, PS 42:8, PS O-41:0, PS 38:4, PS 36:1) and phosphatidylinositol (PI O-39:1) featured prominently among the many species lowering in response to TMZ (Supplementary Desk 1). Phosphatidylserine upregulation is a central function of glioblastoma multiforme32 that’s modulated by TMZ treatment33, together with via ferroptosis34,35,36. The PI3K–AKT is a ubiquitously upregulated pathway in glioblastoma37, which is leveraged by TMZ38.
These knowledge present that TMZ therapy alters tumour lipid composition and distribution, notably by selectively decreasing particular metabolites. Utilizing longitudinal knowledge from molecular replicas, we characterised the response of the tumour and recognized goal lipids beforehand linked to glioma and TMZ. This evaluation provides priceless insights into metabolic pathways affected by the therapy and potential biomarkers of therapeutic efficacy.